Title
题目
Cycle-constrained adversarial denoising convolutional network for PET image denoising: Multi-dimensional validation on large datasets with reader study and real low-dose data
带循环约束的对抗性去噪卷积网络用于PET图像去噪:基于大型数据集、阅片者研究及真实低剂量数据的多维度验证
01
文献速递介绍
正电子发射断层显像(PET)是一种无创成像技术,通过放射性示踪剂可视化代谢和生理过程(Beyer 等,2000;Fletcher 等,2008;Rohren 等,2004;Schwaiger 等,2005;Ter-Pogossian 等,1975)。示踪剂注射后会发生β⁺衰变并释放正电子,正电子与电子湮灭后产生两束近乎反向的伽马射线,这些射线被探测为符合事件,从而为体内功能研究提供依据(Berg 和 Cherry,2018;Cherry 等,2018;El Fakhri 等,2011;Turkington,2001)。然而,示踪剂的放射性具有显著辐射危害,对婴幼儿、儿童和青少年等敏感人群的影响尤为突出(Robbins,2008;Schaefferkoetter 等,2015)。减少 PET 检查中的辐射暴露对于降低放射性示踪剂相关风险至关重要。低剂量 PET 扫描为减轻患者辐射负担提供了可行方案,在母胎医学领域尤为重要——该技术可更安全地研究胎儿和胎盘生理功能,成为传统检查的无创替代方案。 但低剂量 PET 图像易受噪声增多影响,导致图像质量大幅下降。随着辐射剂量降低,噪声愈发明显,遮挡组织和病变结构,进而限制图像的诊断价值(Schaefferkoetter 等,2015)。因此,从低剂量扫描中准确重建全剂量质量图像的方法具有重要临床意义,可在减少辐射暴露与获取准确可靠诊断信息之间实现平衡。此外,在 PET 检查需求较高的地区,受排班限制,患者注射示踪剂后可能需等待一段时间才能成像。这段时间内示踪剂会发生放射性衰变,导致成像时有效剂量自然降低,可能影响图像质量和诊断准确性,对小病变检测的影响尤为显著。这一问题进一步凸显了从低剂量扫描中准确恢复全剂量 PET 图像的临床必要性。 已有多种卷积神经网络(CNN)模型用于低剂量 PET 图像去噪,旨在将低剂量图像恢复至全剂量质量。Jiang 等提出一种半监督模型,对预分割组织应用区域归一化并强化结构一致性,从非配对低剂量 PET 数据中生成标准剂量 PET 图像(Jiang 等,2023)。Jang 等设计一种空间-通道编码-解码Transformer,整合空间和通道信息以增强 PET 图像去噪效果(Jang 等,2023)。Wang 等在六个计数水平上对比了五种先进深度学习模型——U-Net(Ronneberger 等,2015)、增强型深度超分辨率网络(EDSR)(Lim 等,2017)、生成对抗网络(GAN)(Goodfellow 等,2014)、Swin Transformer 网络(SwinIR)(Liang 等,2021)以及带有 EDSR 编码器的视觉Transformer(ViT)(Dosovitskiy,2020)(Wang 等,2023),结果表明 SwinIR 和 U-Net 显著提升了诊断图像质量。然而,大多数模型在降噪的同时牺牲了边缘、小结构等关键细节,往往生成类似传统滤波器处理后的过度平滑图像,导致重要信息丢失(Liu 等,2021,2022)。为解决这一局限,Liu 等提出一种融合策略,结合高噪声和低噪声图像训练模型的输出,在降噪与图像清晰度之间取得平衡(Liu 等,2022)。在医学图像去噪中,核心挑战之一是恢复结构细节(尤其是小病变的形态和活性),因此需要在降噪的同时保留诊断完整性的方法。 CycleGAN(Zhu 等,2017)旨在实现无配对图像的图像转换,可修改特定特征同时保留图像核心特征。该模型采用两个生成器学习域间映射,两个鉴别器区分真实图像与生成图像,通过三种关键损失函数优化:对抗损失使生成图像分布与目标域对齐,循环一致性损失确保输入图像可从转换后的版本重建,身份损失确保图像经原始域对应生成器处理后保持不变。这些损失函数使 CycleGAN 能够完成风格迁移、目标变形和图像增强等任务。Zhou 等将 CycleGAN 应用于 PET 图像去噪,将低剂量和全剂量图像视为不同域(Zhou 等,2020),通过整合对抗损失、循环一致性损失、身份损失和监督损失,在降噪的同时保持了病变对比度。但作为生成模型,CycleGAN 在训练过程中存在不确定性,难以保留细小组织结构和肿瘤轮廓,需要精心设计损失函数以有效约束模型。 去噪扩散概率模型(DDPM)(Ho 等,2020)是近年来兴起的强大生成框架,在多种图像生成任务中表现出色。DDPM 通过学习近似数据分布的扩散过程的逆过程,从高斯噪声中逐步生成目标图像。基于该框架,研究人员开发了多种用于低剂量 PET 去噪的 DDPM 衍生模型,包括 DDPM-PET、DDPM-MR、DDPM-MR-PETCon 和 DDPM-PETMR 等,这些模型基于不同条件先验及其组合设计(Gong 等,2024)。但 DDPM 推理过程中的迭代采样通常计算成本较高。为缩短 DDPM 的推理时间,PET-CM 将一致性模型(Song 等,2023)融入 PET 去噪过程,仅需两步即可生成图像(Pan 等,2024)。此外,BC-DPM 作为一种无监督 DDPM 衍生模型被用于低剂量 PET 图像去噪(Shen 等,2024),其在训练阶段采用原始无条件 DDPM 框架,在采样阶段引入两种手工设计的条件以引导生成过程。尽管取得了这些进展,但基于扩散的模型从训练集中学习经验数据分布,在特定情况下(尤其是遇到罕见肿瘤或非典型解剖结构时)可能引入细微的不一致性或伪影。 本研究提出并深入探讨了一种新型带循环约束的对抗性去噪卷积网络(Cycle-DCN),旨在降低 PET 图像噪声的同时保留诊断完整性。具体而言,本研究的贡献与创新如下: 1. 开发了 Cycle-DCN 模型,该模型通过噪声提取器提取噪声实现域映射,同时整合一致性网络从相邻切片中获取辅助信息。模型采用复合损失函数优化,包括监督损失、对抗损失、循环一致性损失、身份损失和邻域切片结构相似性指数(SSIM)损失,每种损失均有助于保留结构特征和抑制伪影。重要的是,Cycle-DCN 在训练过程中采用动态加权策略,自适应平衡各损失组件,确保其在整个优化过程中有效发挥作用。该方法实现了视觉保真度与去噪性能的良好平衡。此外,通过系统的消融实验,评估了每个网络组件和损失项对整体图像质量的单独贡献。 2. 在中国科学技术大学附属第一医院,使用临床西门子 Biograph PET/CT 扫描仪采集了 1224 例原始脑部 PET 数据集,并通过自定义开发的 QuanTOF 算法(Yuan 等,2024)进行重建。这些复杂的脑部图像作为主要数据集,用于严格评估 Cycle-DCN 在去噪输出中保留精细结构细节的能力。为进一步评估模型在超低剂量条件下的性能,额外采集了 50 例全身 PET 数据集,使用同一扫描仪以 2.2 mm/s 的速度在连续床位运动(CBM)模式下扫描。此外,为评估模型在不同成像中心、患者人群和解剖区域的泛化能力,纳入了北京友谊医院使用西门子 Biograph mCT PET/CT 扫描仪采集的 245 例独立儿科全身 PET 数据集。 3. 采用多维度评估框架评估去噪性能,包括峰值信噪比(PSNR)、结构相似性指数(SSIM)、归一化均方根误差(NRMSE)、对比噪声比(CNR)、边缘保留指数(EPI)和边缘轮廓的豪斯多夫距离等指标。将多种经典去噪模型和近期提出的基于扩散的方法作为基线对比,并综合分析其优势与局限性。此外,来自多家医院的 5 名核医学医师(均拥有 10 年以上经验)对去噪后的图像进行了评估和评分。该综合评估验证了模型在降噪、保留脑沟回结构以及恢复肿瘤形态和对比度方面的有效性。
Aastract
摘要
Positron emission tomography (PET) is a critical tool for diagnosing tumors and neurological disorders but poses radiation risks to patients, particularly to sensitive populations. While reducing injected radiation dose mitigates this risk, it often compromises image quality. To reconstruct full-dose-quality images from low-dose scans, we propose a Cycle-constrained Adversarial Denoising Convolutional Network (Cycle-DCN). This model integrates a noise predictor, two discriminators, and a consistency network, and is optimized using a combination of supervised loss, adversarial loss, cycle consistency loss, identity loss, and neighboring Structural Similarity Index (SSIM) loss. Experiments were conducted on a large dataset consisting of raw PET brain data from 1224 patients, acquired using a Siemens Biograph Vision PET/CT scanner. Each patient underwent a 120-seconds brain scan. To simulate low-dose PET conditions, images were reconstructed from shortened scan durations of 30, 12, and 5 s, corresponding to ¼, ⅒, and 1/24 of the full-dose acquisition, respectively, using a custom-developed GPUbased image reconstruction software. The results show that Cycle-DCN significantly improves average Peak Signal-to-Noise Ratio (PSNR), SSIM, and Normalized Root Mean Square Error (NRMSE) across three dose levels, with improvements of up to 56%, 35%, and 71%, respectively. Additionally, it achieves contrast-to-noise ratio (CNR) and Edge Preservation Index (EPI) values that closely align with full-dose images, effectively preserving image details, tumor shape, and contrast, while resolving issues with blurred edges. The results of reader studies indicated that the images restored by Cycle-DCN consistently received the highest ratings from nuclear medicine physicians, highlighting their strong clinical relevance. A separate set of 50 whole-body PET datasets acquired using the same Biograph Vision scanner, along with an independent set of 245 whole-body pediatric PET datasets acquired using a Siemens Biograph mCT PET/CT scanner at Beijing Friendship Hospital, further validate the generalizability of the proposed model across different imaging centers, scanner types, scanning mode, patient demographics, and anatomical regions.
正电子发射断层显像(PET)是诊断肿瘤和神经系统疾病的关键工具,但会给患者带来辐射风险,对敏感人群的影响尤为显著。降低注射辐射剂量虽能减轻这一风险,却往往会损害图像质量。为从低剂量扫描中重建出全剂量质量的图像,我们提出一种带循环约束的对抗性去噪卷积网络(Cycle-DCN)。该模型整合了噪声预测器、两个鉴别器和一个一致性网络,并通过监督损失、对抗损失、循环一致性损失、身份损失及邻域结构相似性指数(SSIM)损失的组合进行优化。 实验基于大型数据集开展,该数据集包含1224名患者的原始脑部PET数据,由西门子Biograph Vision PET/CT扫描仪采集,每位患者均接受了120秒的脑部扫描。为模拟低剂量PET场景,我们使用自定义开发的基于GPU的图像重建软件,从缩短至30秒、12秒和5秒的扫描时长中重建图像,分别对应全剂量采集的¼、⅒和1/24。 结果显示,Cycle-DCN在三个剂量水平下均显著提升了平均峰值信噪比(PSNR)、结构相似性指数(SSIM)和归一化均方根误差(NRMSE),最大提升幅度分别达56%、35%和71%。此外,该模型生成的图像在对比噪声比(CNR)和边缘保留指数(EPI)上与全剂量图像高度吻合,能有效保留图像细节、肿瘤形态和对比度,同时解决了边缘模糊问题。阅片者研究结果表明,经Cycle-DCN修复的图像持续获得核医学医师的最高评分,凸显其较强的临床相关性。 另一组包含50例使用同一Biograph Vision扫描仪采集的全身PET数据集,以及北京友谊医院使用西门子Biograph mCT PET/CT扫描仪采集的245例独立儿科全身PET数据集,进一步验证了所提模型在不同成像中心、扫描仪类型、扫描模式、患者人群和解剖区域的泛化能力。
Method
方法
2.1. PET image reconstruction
In this study, 1224 head scan datasets were acquired using a Siemens Biograph Vision PET/CT scanner. The raw list-mode datasets were reconstructed using QuanTOF (Yuan et al., 2024), a GPU-accelerated, Bayesian penalized likelihood reconstruction algorithm. QuanTOF integrates time-of-flight (TOF) information and complete correction techniques, ensuring high-quality PET image reconstruction. To simulate low-dose PET conditions, images were reconstructed from scan shortened durations of 30, 12, and 5 s, corresponding to ¼, ⅒, and 1/24 of the full dose, respectively, with 120 s of scan data serving as the full-dose reference. As illustrated in Fig. 1, the reconstructed images via QuanTOF using list-mode datasets, due to accurate modeling of imaging physics, exhibit clearer details and sharper edges compared to those reconstructed via the vendor’s e7-tools solution using sinogram datasets, providing a more reliable assessment of the model’s ability to preserve fine details.
2.1 PET图像重建 本研究使用西门子Biograph Vision PET/CT扫描仪采集了1224例头部扫描数据集。原始列表模式数据集通过QuanTOF算法(Yuan等,2024)进行重建,该算法是一种基于GPU加速的贝叶斯惩罚似然重建算法。QuanTOF整合了飞行时间(TOF)信息和完整的校正技术,确保了高质量的PET图像重建。为模拟低剂量PET场景,研究从缩短至30秒、12秒和5秒的扫描时长中重建图像,分别对应全剂量的¼、⅒和1/24,以120秒的扫描数据作为全剂量参考。如图1所示,基于列表模式数据集通过QuanTOF重建的图像,由于对成像物理过程的精确建模,与通过厂商e7-tools方案基于正弦图数据集重建的图像相比,细节更清晰、边缘更锐利,为评估模型保留精细细节的能力提供了更可靠的依据。
Conclusion
结论
In this study, we present a Cycle-constrained Adversarial Denoising Convolutional Network (Cycle-DCN) designed to effectively reduce noise while preserving image clarity and ensuring the comprehensive restoration of fine structural features, such as brain sulci and the shape and activity of small lesions. The model was trained and tested on 1224 datasets acquired using a Siemens Biograph Vision PET/CT scanner. Test results demonstrate that Cycle-DCN enhances the average PSNR, SSIM, and NRMSE values across three dose levels by up to 56%, 35%, and 71%, respectively. It successfully restores tumor shapes at all three low-dose levels and achieves tumor contrast that closely resembles full-dose images. Additionally, Cycle-DCN excels in accurately restoring the structures of brain sulci and gyri, closely mirroring those in full-dose images. The images produced exhibit an optimal Edge Preservation Index (EPI) and the smallest Hausdorff Distance for edges extracted via the Canny operator when compared to full-dose images. The external validation using CBM scan data and pediatric data demonstrated the generalizability of the proposed model across different imaging centers, scanner types, scanning mode, patient demographics, and anatomical regions. Furthermore, the reader study by five nuclear medicine physicians indicates that our model’s denoised images are the most similar to fulldose images. The physicians expressed a preference for the Cycle-DCN denoised images over those processed with Gaussian filtering or vendor’s e7-tools reconstruction, underscoring the strong clinical significance of our model.While our proposed model has demonstrated favorable outcomes in brain PET image denoising, we are committed to expanding our data sources, adapting the model for various organs and radiotracers, and optimizing the model architecture to create a denoising solution applicable to whole-body PET imaging, thereby providing clearer and more accurate imaging support for clinical diagnosis.
本研究提出一种循环约束对抗性去噪卷积网络(Cycle-DCN),旨在有效降噪的同时保留图像清晰度,确保脑沟、小病灶形态及活性等精细结构特征的全面恢复。该模型基于西门子Biograph Vision PET/CT扫描仪采集的1224个数据集进行训练和测试。测试结果显示,Cycle-DCN在三种剂量水平下,将平均峰值信噪比(PSNR)、结构相似性指数(SSIM)和归一化均方根误差(NRMSE)分别提升最高达56%、35%和71%。模型成功在所有三种低剂量水平下恢复了肿瘤形态,且实现的肿瘤对比度与全剂量图像高度接近。此外,Cycle-DCN在精准恢复脑沟、脑回结构方面表现优异,与全剂量图像中的对应结构高度吻合。经Canny算子提取边缘后,该模型生成的图像具有最优的边缘保留指数(EPI),且与全剂量图像的豪斯多夫距离最小。通过连续床位运动(CBM)扫描数据和儿科数据进行的外部验证表明,所提模型在不同成像中心、扫描仪类型、扫描模式、患者人群及解剖区域均具有良好的泛化能力。此外,五位核医学医师参与的阅片研究显示,该模型的去噪图像与全剂量图像最为相似。医师们更偏好Cycle-DCN的去噪结果,而非高斯滤波或厂商e7-tools重建处理的图像,凸显了该模型强大的临床意义。 尽管所提模型在脑部PET图像去噪中已展现出良好效果,我们仍致力于拓展数据来源,将模型适配于不同器官和放射性示踪剂,并优化模型架构,打造适用于全身PET成像的去噪方案,为临床诊断提供更清晰、准确的成像支持。
Results
结果
4.1. Quantitative evaluations
4.1.1. PSNR, SSIM, and NRMSE comparison
The proposed Cycle-DCN model was systematically compared with several established image denoising and restoration methods, including widely used U-Net, classic denoising model DnCNN (Zhang et al., 2017), the more recent image restoration model CGNet (Ghasemabadi et al., 2024), the Swin Transformer-based SwinIR, CycleGAN, and three diffusion-based approaches: DDPM-PET, PET-CM, and BC-DPM. Table 1provides the quantitative results, showing the mean, standard deviation, and statistical significance based on paired t-tests for each evaluation metric. Fig. 4(a) presents a visual comparison of a representative slice across the nine models, along with the corresponding full-dose and low-dose images at three different dose levels.For ¼ low-dose images, the PSNR, SSIM, and NRMSE metrics are comparable across all evaluated models. While DnCNN and SwinIR slightly outperforms Cycle-DCN in some measures, these differences are not statistically significant (p > 0.05). In contrast, for the more challenging 1/24 low-dose images, Cycle-DCN achieves superior metric values. The three diffusion-based methods—DDPM-PET, PET-CM, and BC-DPM—consistently yielded inferior performance under all the three low-dose conditions. As shown in Fig. 4(a), U-Net and DnCNN result in excessive smoothing, leading to increased deviation from the full-dose images at lower dose levels, with small sulci becoming nearly indistinguishable. However, Cycle-DCN remains closer to the full-dose images, demonstrating better robustness. In the enlarged views in Fig. 4(b), Cycle-DCN uniquely preserves brain structures such as sulci and gyri, producing visual outputs more consistent with full-dose images compared to other models. Among the three diffusion-based methods, DDPM-PET produced visually promising results across all dose levels. However, the performance of BC-DPM (trained unconditionally) and PET-CM (with two-step sampling) deteriorated rapidly as the dose decreased, resulting in significantly degraded image quality.
4.1 定量评估 4.1.1 峰值信噪比(PSNR)、结构相似性指数(SSIM)及归一化均方根误差(NRMSE)对比 将所提Cycle-DCN模型与多种已有的图像去噪和恢复方法进行了系统对比,包括广泛使用的U-Net、经典去噪模型DnCNN(Zhang等,2017)、较新的图像恢复模型CGNet(Ghasemabadi等,2024)、基于Swin Transformer的SwinIR、CycleGAN,以及三种基于扩散的方法(DDPM-PET、PET-CM和BC-DPM)。表1给出了定量结果,包括各评估指标的均值、标准差以及基于配对t检验的统计显著性。图4(a)展示了九种模型在三个不同剂量水平下,代表性切片的视觉对比,同时呈现了对应的全剂量和低剂量图像。 对于¼低剂量图像,所有评估模型的PSNR、SSIM和NRMSE指标表现相当。尽管DnCNN和SwinIR在部分指标上略优于Cycle-DCN,但这些差异无统计学意义(p > 0.05)。相比之下,在更具挑战性的1/24低剂量图像中,Cycle-DCN取得了更优的指标值。三种基于扩散的方法(DDPM-PET、PET-CM和BC-DPM)在所有三个低剂量条件下均表现出持续劣势。如图4(a)所示,U-Net和DnCNN存在过度平滑问题,导致在低剂量水平下与全剂量图像的偏差增大,细小脑沟几乎无法区分。而Cycle-DCN始终更接近全剂量图像,展现出更强的鲁棒性。在图4(b)的放大视图中,Cycle-DCN独特地保留了脑沟、脑回等脑部结构,生成的视觉结果比其他模型更接近全剂量图像。在三种基于扩散的方法中,DDPM-PET在所有剂量水平下均产生了视觉上较理想的结果,但无监督训练的BC-DPM和两步采样的PET-CM的性能随剂量降低迅速下降,导致图像质量显著退化。
Figure
图
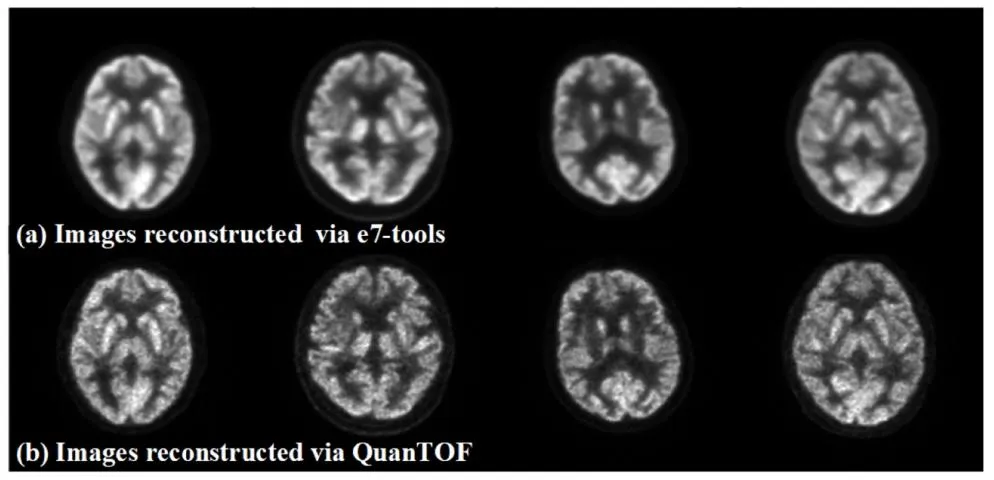
Fig. 1. Images reconstructed by e7-tools (top row) and QuanTOF (bottom row).
图1 分别通过e7-tools(上行)和QuanTOF(下行)重建的图像。

Fig. 2. Diagrams of the Cycle-DCN model. (a) Noise extraction process; (b), © Noise removal and addition processes; (d), (e) Generation of the same and recovered images; (f) Composition of the Cycle-DCN loss function.
图2 Cycle-DCN模型示意图。(a) 噪声提取过程;(b)、© 噪声去除与添加过程;(d)、(e) 相同图像与恢复图像的生成过程;(f) Cycle-DCN损失函数的构成。

Fig. 3. Network architecture diagrams for the (a) Noise Predictor Network and Consistency Network; (b) Discriminator.
图3 网络架构示意图。(a) 噪声预测器网络与一致性网络架构;(b) 鉴别器网络架构。
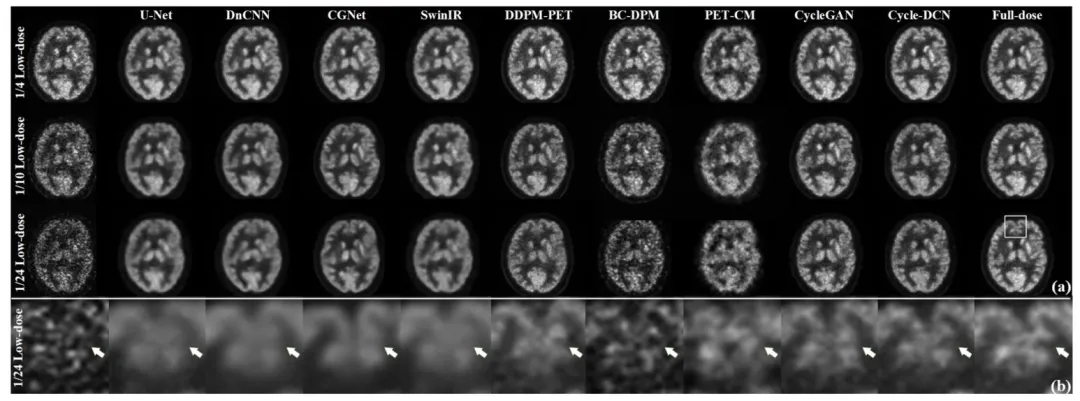
Fig. 4. (a) Denoised images from various models at three different low-dose levels for a representative case; (b) The partially enlarged sections of 1/24 low-dose images, showcasing detailed visual comparisons across models.
图4 (a) 代表性病例在三种不同低剂量水平下,各模型的去噪图像;(b) 1/24低剂量图像的局部放大图,展示各模型间的细节视觉对比。

Fig. 5. Denoised images from various models at three low-dose levels for two representative cases.
图5 两个代表性病例在三种低剂量水平下,各模型的去噪图像。

Fig. 6. The selected tumor and background regions.
图6 选定的肿瘤区域与背景区域。

Fig. 7. The edge-extracted images obtained using the Canny operator.
图7 采用Canny算子提取的边缘图像。
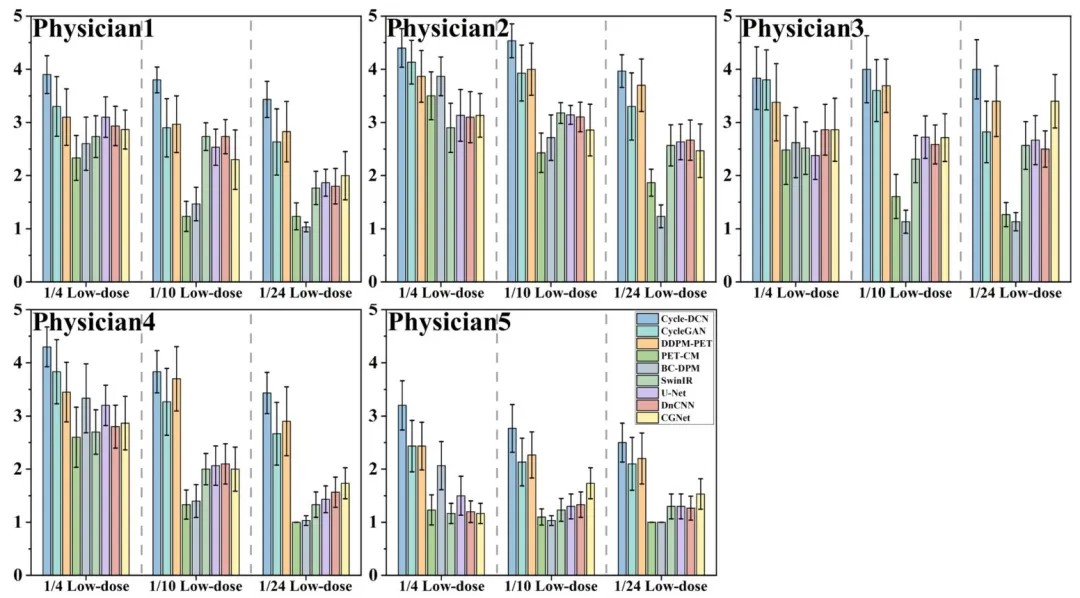
Fig. 8. The scoring results from the five physicians for different models on the randomly selected 30 cases in the test dataset.
图8 五位医师对测试数据集中随机选取的30个病例,针对不同模型的评分结果。
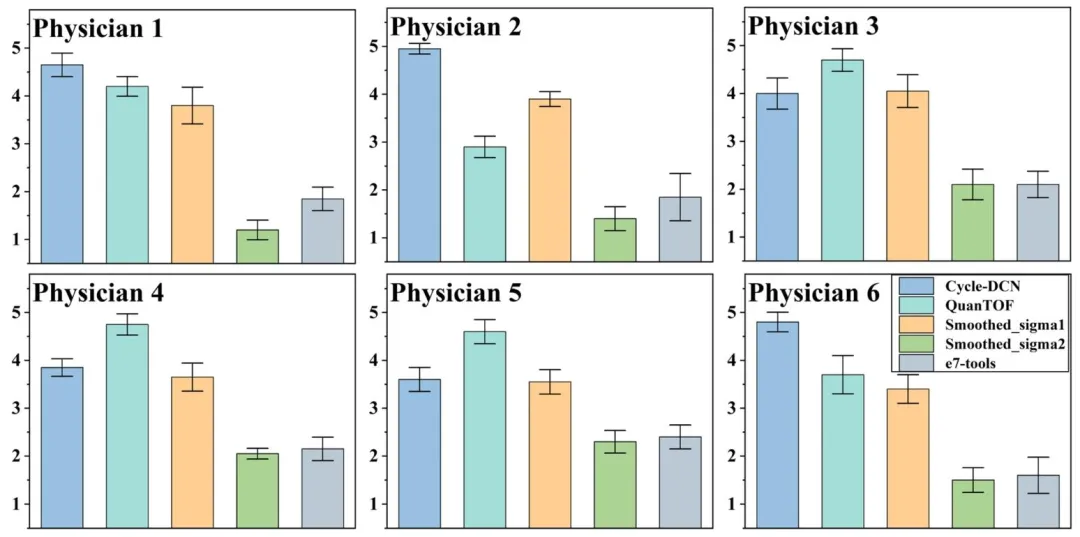
Fig. 9. The scoring results for real low-dose images processed using different algorithms on the randomly selected 20 cases in the test dataset.
图9 五位医师对测试数据集中随机选取的20个病例,经不同算法处理后的真实低剂量图像的评分结果。

Fig. 10. Denoising results of real low-dose images using different methods (top row) and the enlarged sections (bottom row) for one representative case
图10 一个代表性病例的真实低剂量图像经不同方法处理后的去噪结果(上行)及局部放大图(下行)

Fig. 11. Horizontal and coronal slices of denoised images from different models at two low-dose levels for a representative case.
图11 一个代表性病例在两种低剂量水平下,不同模型去噪图像的水平位切片与冠状位切片。

Fig. 12. Horizontal and coronal slices of denoised images from different models at two low-dose levels for a representative case
图12 一个代表性病例在两种低剂量水平下,不同模型去噪图像的水平位切片与冠状位切片。
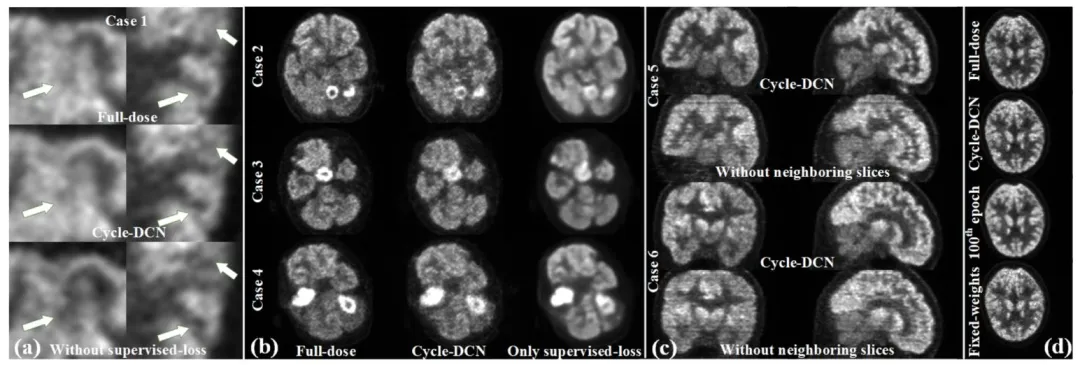
Fig. 13. (a) Comparison of the enlarged full-dose images, denoised images using Cycle-DCN, and denoised images from the model without supervised loss; (b) Comparison of the full-dose image, denoised images from Cycle-DCN, and denoised image from the model using only supervised loss for 3 different cases; © Comparison of denoised images in coronal (left column), and sagittal planes (right column) for two representative cases with/without neighboring slices assisted; (d) Denoised images under different loss weighting strategies.
图13 (a) 全剂量图像放大图、Cycle-DCN去噪图像与无监督损失模型去噪图像的对比;(b) 3个不同病例的全剂量图像、Cycle-DCN去噪图像与仅使用监督损失模型去噪图像的对比;© 两个代表性病例在有无相邻切片辅助情况下,冠状位(左列)和矢状位(右列)去噪图像的对比;(d) 不同损失权重策略下的去噪图像。

Fig. 14. (a) Training loss curves using dynamic loss weighting; (b) Training loss curves using fixed loss weighting; © Corresponding test loss curves
图14 (a) 采用动态损失权重的训练损失曲线;(b) 采用固定损失权重的训练损失曲线;© 对应的测试损失曲线。
Table
表
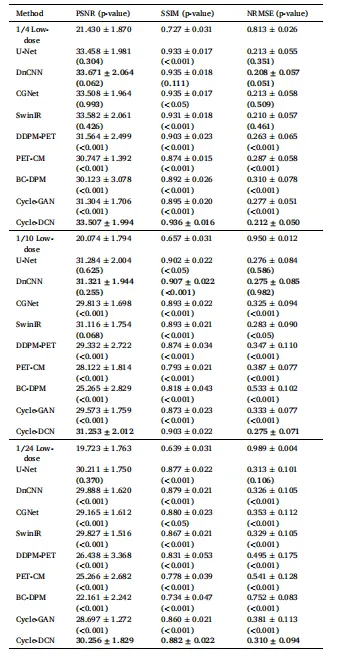
Table 1 Quantitative Comparison of Mean PSNR, SSIM and NRMSE among DifferentModels on the Test Dataset.
表1 不同模型在测试数据集上的平均峰值信噪比(PSNR)、结构相似性指数(SSIM)及归一化均方根误差(NRMSE)定量对比
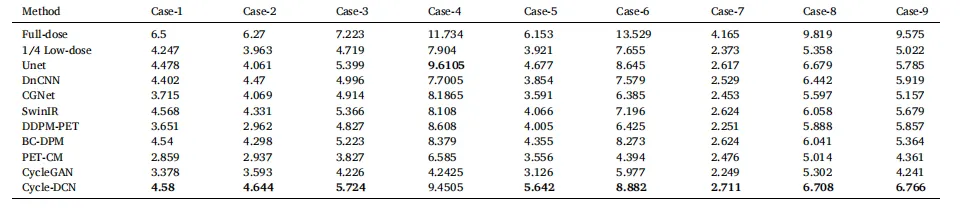
Table 2 Comparison of CNR across 9 Cases for Different Models
表2 不同模型在9个病例中的对比噪声比(CNR)对比
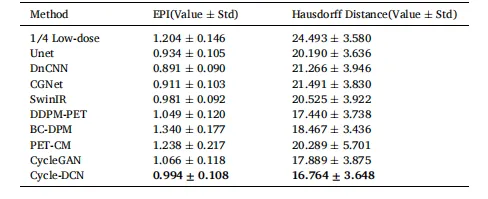
Table 3 Comparison of EPI and Hausdorff Distance for Different Models(Value ± Std).
表3 不同模型的边缘保留指数(EPI)与豪斯多夫距离对比(数值±标准差)
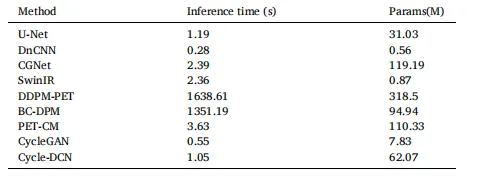
Table 4 Inference Time and Parameters of Different Models
表4 不同模型的推理时间与参数对比

Table 5 Quantitative Comparison of Mean PSNR, SSIM and NRMSE on the whole-body CBM datasets.
表5 全身连续床位运动(CBM)数据集上的平均峰值信噪比(PSNR)、结构相似性指数(SSIM)及归一化均方根误差(NRMSE)定量对比

Table 6 Quantitative Comparison of Mean PSNR, SSIM and NRMSE on the whole-body pediatric datasets.
表6 全身儿科数据集上的平均峰值信噪比(PSNR)、结构相似性指数(SSIM)及归一化均方根误差(NRMSE)定量对比

Table 7 Quantitative Comparison of Models with Different Loss and Components (Value ± Std).
表7 不同损失函数与组件的模型定量对比(数值±标准差)