Title
题目
IterMask3D: Unsupervised anomaly detection and segmentation withtest-time iterative mask refinement in 3D brain MRI
IterMask3D:基于测试时迭代掩码优化的3D脑部磁共振成像(MRI)无监督异常检测与分割
01
文献速递介绍
医学图像分析中异常分割相关研究(IterMask3D方法背景与设计) 在医学图像分析领域,异常分割至关重要,因其可支持早期疾病检测与诊断、指导治疗方案制定,并减轻临床工作负担。 传统异常分割方法多为有监督学习,依赖带标注的训练数据——即图像中需包含异常区域,且对应区域需人工标注类别。这类方法在有限数据类型上训练后,仅能分割与训练数据中相似的异常,难以检测其他未见过的异常类型。在本研究聚焦的脑部磁共振成像(MRI)场景中,此类方法通常仅针对特定病症(Kamnitsas 等,2017;Isensee 等,2021;Stollenga 等,2015)。 与之相反,无监督异常分割在训练过程中无需任何“异常图像”及其人工分割标注。该方法仅用“正常”图像训练模型,并将训练数据的分布作为“正常”参考标准。测试时,凡偏离此参考标准的模式均被视为“异常”,并尝试对其进行分割。这种特性使其能够检测此前未见过的模式(包括新型病症甚至成像伪影),因此适用于偶然发现自动筛查(Rowley 等,2023)或扫描质量自动控制(Hendriks 等,2024)等任务。当训练数据有限、且提前未知具体病症类型时,该方法优势尤为突出,有助于罕见病的检测。本研究即围绕无监督异常分割展开,重点针对脑部MRI数据。 学习训练数据分布的主流策略是基于重建的方法,这类方法遵循“损坏-重建”范式。训练阶段,先对图像进行人工损坏处理,再训练模型重建原始的“正常”分布内图像,模型通过重建过程逐步内化正常分布。测试时,偏离已学习正常分布的区域(即异常区域)往往会产生较高的重建误差,这些误差可用于异常分割。常见的损坏方式包括压缩(Atlason 等,2019;Baid 等,2021)或扩散模型中的噪声添加(Pinaya 等,2022a;Bercea 等,2023a;Wolleb 等,2022),模型的任务便是从这些损坏图像中重建出原始正常图像。 此类方法普遍面临“灵敏度-精度权衡”问题(如图1所示):为放大异常区域的重建误差以实现更好的分割效果,需增加损坏程度;但由于异常位置提前未知,损坏处理会作用于整个图像——这不仅会增大异常区域的重建误差,也会提高正常区域的重建误差,进而导致假阳性(将正常误判为异常)。如图1所示,提高噪声强度和压缩程度虽会增大异常区域(此处为高信号肿瘤)的重建误差,有助于更好地检测异常区域,但同时也会使正常区域的重建误差增大,引发假阳性。 这一“灵敏度-精度权衡”问题正是本研究的核心动机:能否通过输入损坏处理放大异常区域的重建误差以优化分割效果,同时保留正常区域的信息以最小化其重建误差、降低假阳性率? 为解决该问题,我们提出适用于3D MRI数据的无监督异常分割与检测迭代式掩码优化方法——IterMask3D。为放大异常区域的重建误差,我们引入空间掩码作为一种损坏形式;为最小化正常区域的误差,我们采用迭代式空间掩码缩小流程,逐步将掩码向异常区域收敛,以减少信息丢失。随着正常组织被逐步解除掩码并输入模型,其信息会指导掩码下正常结构的重建,从而降低假阳性率。此外,我们通过高频掩码生成结构图像,为掩码缩小流程提供结构指导,以优化正常区域的重建效果。 本研究的主要贡献如下: 1. 提出3D无监督异常分割与检测方法IterMask3D,该方法通过将空间掩码向异常区域迭代缩小,降低正常区域的假阳性率。 2. 进一步提出利用频率掩码生成高频图像,将其作为掩码缩小流程的结构指导,提升正常区域的重建效果,进一步降低假阳性率。 3. 引入特定于被试的阈值策略:在每次迭代中确定误差图上哪些区域可被可靠地归类为正常。该策略使迭代式掩码缩小流程能为每个被试自动确定最优停止点,无需从验证数据中获取额外信息。 4. 在多种异常类型(包括病理性异常和伪影类异常)上验证了所提方法的有效性。具体而言,我们在合成与真实成像伪影、以及多种MRI序列中的肿瘤和中风等病症上对模型进行了测试。 本研究基于我们此前的工作(Liang 等,2024)展开,该工作中我们提出了用于2D脑部MRI病症无监督异常分割的迭代式掩码优化方法(IterMask2D)。此前的2D模型遵循异常分割的标准流程:在健康图像切片上训练,在同一数据集的异常切片上评估。本研究在其基础上进行了如下拓展: a) 将方法扩展至3D领域,并在来自不同分布的真实训练与测试数据上评估,进一步提升其在真实场景中的适用性; b) 将评估范围从病理性异常拓展至成像伪影,验证模型对两类异常的检测效果; c) 摒弃针对整个数据集的固定阈值,采用特定于图像的阈值策略,自动确定掩码缩小流程的停止时机——无需依赖任何验证数据; d) 此前的方法需两个独立模型:一个用于掩码覆盖全脑区域时的初始预测,另一个用于后续的掩码缩小流程;本研究将这些步骤整合到单一模型中,实现全流程统一; e) 扩展掩码优化流程,使其同时支持缩小与扩展,提升灵活性并能修正前期步骤中的误差。 论文其余部分结构如下:第2节介绍相关工作;第3节阐述所提方法IterMask3D;第4节说明实验设置;第5节呈现实验结果,涵盖四项任务——合成伪影检测(5.1节)、真实伪影检测(5.2节)、2D数据病症分割(5.3节)及3D数据病症分割(5.4节);最后,第6节进行讨论并总结全文。
Aastract
摘要
Unsupervised anomaly detection and segmentation methods train a model to learn the training distribution as‘normal’. In the testing phase, they identify patterns that deviate from this normal distribution as ‘anomalies’.To learn the ‘normal’ distribution, prevailing methods corrupt the images and train a model to reconstructthem. During testing, the model attempts to reconstruct corrupted inputs based on the learned ‘normal’distribution. Deviations from this distribution lead to high reconstruction errors, which indicate potentialanomalies. However, corrupting an input image inevitably causes information loss even in normal regions,leading to suboptimal reconstruction and an increased risk of false positives. To alleviate this, we proposeIterMask3D, an iterative spatial mask-refining strategy designed for 3D brain MRI. We iteratively spatially maskareas of the image as corruption and reconstruct them, then shrink the mask based on reconstruction error.This process iteratively unmasks ‘normal’ areas to the model, whose information further guides reconstructionof ‘normal’ patterns under the mask to be reconstructed accurately, reducing false positives. In addition, toachieve better reconstruction performance, we also propose using high-frequency image content as additionalstructural information to guide the reconstruction of the masked area. Extensive experiments on the detectionof both synthetic and real-world imaging artifacts, as well as segmentation of various pathological lesionsacross multiple MRI sequences, consistently demonstrate the effectiveness of our proposed method.
无监督异常检测与分割方法相关研究(IterMask3D方法介绍) 无监督异常检测与分割方法通过训练模型,将训练数据的分布学习为“正常”分布。在测试阶段,这些方法会把偏离该正常分布的模式识别为“异常”。 为学习“正常”分布,主流方法会对图像进行损坏处理,再训练模型对损坏图像进行重建。测试时,模型会基于已学习到的“正常”分布,尝试对损坏的输入图像进行重建。若图像偏离该正常分布,会导致较高的重建误差,而这种高误差则意味着存在潜在异常。 然而,对输入图像进行损坏处理时,即便在正常区域也不可避免地会造成信息丢失,这不仅会导致重建效果欠佳,还会增加假阳性(误判为异常)的风险。为缓解这一问题,我们提出了适用于3D脑部磁共振成像(MRI)的迭代式空间掩码优化策略——IterMask3D。该策略通过以下方式运作:首先,迭代性地对图像局部区域进行空间掩码处理(将掩码区域视为损坏区域)并对其进行重建;随后,根据重建误差缩小掩码范围。 这一过程会逐步将“正常”区域从掩码中释放(即解除对正常区域的掩码),这些正常区域的信息会进一步指导模型对仍处于掩码下的区域进行“正常”模式重建,从而实现精准重建,降低假阳性率。此外,为实现更优的重建性能,我们还提出将图像的高频内容作为额外的结构信息,用于指导掩码区域的重建。 我们在多个磁共振成像序列上开展了大量实验,实验内容既包括对合成及真实成像伪影的检测,也涵盖对各类病理性病变的分割。实验结果一致表明,我们提出的方法具有显著有效性。
Method
方法
3.1. The overview
Our proposed method is a reconstruction-based unsupervised anomaly segmentation method. The training dataset, 𝐷**𝑡𝑟 = { 𝐱 𝑖 }𝑁𝑖*=1, consists of ‘normal’ in-distribution samples. The model aims to learn thetraining distribution following the ‘corrupt and reconstruct’ paradigmas previously introduced in Section 1, which learns to reconstructin-distribution samples after corruption. During testing, given testingdataset 𝐷**𝑡𝑒 = { 𝐱 𝑖 }𝑀𝑖*=1, the model aims to segment any patterns outsidethe training distribution (unseen during training) as anomalies. To mitigate the sensitivity-precision trade-off in reconstruction-based methods,we propose an iterative spatial mask refinement process, 𝐼 𝑡𝑒𝑟𝑀𝑎𝑠𝑘3𝐷,as shown in Fig. 2© Testing Process. To ensure large reconstructionerrors in abnormal regions for effective anomaly segmentation, spatialmasking is applied to completely mask anomalies before reconstruction.Additionally, to accurately reconstruct normal areas with minimal reconstruction error, we gradually shrink the mask towards the anomaly.This process progressively unmasks normal regions, providing the network with more information on case-specific ‘normal’ patterns to betterreconstruct normal areas under the mask.Specifically, as shown in Fig. 2© Iterative Mask Refinement, westart by masking the entire brain area. In each iteration, the network reconstructs the masked regions, and areas with small errors areidentified as normal and subsequently unmasked. With every iteration, more information is revealed, allowing the network to refine itsreconstruction.However, as the network is guided only by the unmasked regions,it must infer the missing structures in a generative manner. Incorrectly inferred structures will introduce reconstruction errors evenin normal areas, leading to false positives. To address this, we further propose incorporating high-frequency information (achieved vialow-frequency masking in the Fourier space) to guide the structuralreconstruction of masked regions, as shown in Fig. 2(a). In each iteration, the threshold for identifying normal regions is decided by theSubject-specific thresholding* strategy, as shown in Fig. 2(d). As the maskis progressively shrunk by a fixed proportion, the threshold is selectedbased on the characteristic change in threshold values throughout themask-shrinking process.Finally, the training process is depicted in Fig. 2(b). To train thereconstruction model, we apply randomly generated Gaussian masks asspatial masks to the input image and combine them with input of highfrequency components (generated as shown in Fig. 2(a)). The model istrained to reconstruct the masked regions using this combined input. Inthe following sections, we introduce Iterative Spatial Mask Refinement(Fig. 2©) in Section 3.2, frequency masking (Fig. 2(a)) in Section 3.3,subject-specific thresholding strategy (Fig. 2(d)) in Section 3.4, and thetraining process (Fig. 2(b)) in Section 3.5.
3.1 方法概述 本文提出的方法是一种基于重建的无监督异常分割方法。训练数据集(D{tr}={x_i}{i=1}N)由“正常”的分布内样本构成。模型旨在遵循第1节中介绍的“损坏-重建”范式来学习训练数据分布,即学习对损坏后的分布内样本进行重建。 测试阶段,给定测试数据集(D{te}={x_i}{i=1}M),模型旨在将训练分布之外(训练过程中未见过)的所有模式分割为异常。为缓解基于重建的方法中存在的“灵敏度-精度权衡”问题,我们提出了一种迭代式空间掩码优化流程——IterMask3D,如图2(c)“测试流程”所示。 为确保异常区域产生较大重建误差,从而实现有效的异常分割,在重建前会采用空间掩码对异常区域进行完全掩盖。此外,为精准重建正常区域并将其重建误差降至最低,我们会逐步将掩码向异常区域收敛。这一过程会逐步解除对正常区域的掩码,为网络提供更多特定病例的“正常”模式信息,从而更好地重建掩码下的正常区域。 具体而言,如图2(c)“迭代式掩码优化”所示,我们首先对全脑区域进行掩码处理。在每次迭代中,网络会对掩码区域进行重建,将误差较小的区域判定为正常区域,并随后解除对这些区域的掩码。每迭代一次,就会有更多信息被揭示,网络得以优化其重建效果。 然而,由于网络仅受未掩码区域的引导,它必须以生成式方式推断缺失的结构。即便在正常区域,推断错误的结构也会引入重建误差,进而导致假阳性。为解决这一问题,我们进一步提出融入高频信息(通过在傅里叶空间中进行低频掩码实现),以指导掩码区域的结构重建,如图2(a)所示。 在每次迭代中,判定正常区域的阈值由“特定于被试的阈值设定”策略确定,如图2(d)所示。当掩码按固定比例逐步缩小时,会根据整个掩码缩小过程中阈值的特征变化来选择最终阈值。 最后,训练流程如图2(b)所示。为训练重建模型,我们将随机生成的高斯掩码作为空间掩码应用于输入图像,并将其与高频成分输入(生成方式如图2(a)所示)相结合。模型通过这种组合输入进行训练,以实现对掩码区域的重建。 在后续章节中,3.2节将介绍迭代式空间掩码优化(图2(c)),3.3节介绍频率掩码(图2(a)),3.4节介绍特定于被试的阈值设定策略(图2(d)),3.5节介绍训练流程(图2(b))。
Conclusion
结论
This work has presented IterMask3D, an unsupervised anomalysegmentation framework developed for 3D brain MRI. By introducingan iterative spatial mask refinement strategy combined with highfrequency structural guidance, IterMask3D effectively detects reconstruction errors in anomalous regions while minimizing false positivesin normal tissue. Additionally, we proposed a subject-specific thresh*olding strategy enabling adaptive anomaly detection, alleviating dependence on thresholds entirely determined using in-distribution validation datasets. Through extensive evaluations on multiple datasets,including synthetic and real-world imaging artifacts, as well as multiple brain pathologies across MRI sequences, IterMask3D consistentlydemonstrated superior or competitive performance compared to previous methods.The promising performance across extensive evaluations underscores the potential of our method. However, several challengesremain—many of which are common in the current literature—andthese point out valuable directions for future work. A common challenge for reconstruction-based anomaly detection methods is the reduced sensitivity to anomalies that produce only subtle reconstructionerrors. Such examples are small artifacts or patterns with average (gray)intensities. An inherent challenge that exacerbates this issue is potentialdomain gap between training and testing datasets (differences in theirimaging characteristics). It is often impractical to train the model ondata obtained from the exact same imaging center or scanner as thetesting data. Although our iterative mask refinement approach progressively reduces this domain gap by iteratively exposing to the modellarger regions from the test image at inference time, anomalies thatgenerate reconstruction errors similar to those caused by domain shiftscan be mistakenly considered normal. Consequently, these anomaliesmay become unmasked and remain undetected during the iterativeprocess.Another challenge is that medical imaging data, especially MRI,differ significantly from natural images in terms of intensity rangevariability. While natural images typically have a fixed intensity range(e.g. 0–255), intensity ranges in MRI images exhibit substantial variability across datasets, scanners and acquisition protocols, as MRIintensity units are not standardized. Large intensity shift due to thisbetween the training and test distribution can lead to suboptimalbehavior of anomaly segmentation methods. A related challenge isthe normalization of medical image intensities, which aims to assigncomparable intensity ranges to the same tissue across images, so thatintensity shifts do not negatively affect reconstruction accuracy andanomaly detection. MRI intensity normalization is still an active field ofresearch, and current normalization methods can be severely affectedby the presence of anomalies in images. Consequently, suboptimalintensity normalization can lead to further challenges for anomalydetection or segmentation.Despite these challenges, our approach, IterMask3D, makes a meaningful contribution to the field by considering the sensitivity–precisiontrade-off and introducing iterative mask shrinking as a means to mitigate it in 3D anomaly detection. Results demonstrate particularlypromising performance for detection of imaging artifacts which couldbe of practical usefulness for quality-assurance in imaging workflows.Results on unsupervised pathology segmentation, although achievingstate-of-the-art for unsupervised segmentation, they still have room forimprovement towards levels achievable by supervised segmentationmethods. The improvement demonstrated by this method, however,shows there is substantial potential in unsupervised anomaly segmentation that is still untapped. Given that this category of methods couldunlock applications for which supervised methods are less appropriate,such as segmentation of any type of brain lesion or incidental findings, we hope this study will motivate further future research in thisdirection.
研究内容与展望 本文提出了IterMask3D——一种专为3D脑部磁共振成像(MRI)设计的无监督异常分割框架。该框架通过引入迭代式空间掩码优化策略,并结合高频结构引导,能够有效检测异常区域的重建误差,同时最大限度减少正常组织中的假阳性。此外,我们还提出了特定于被试的阈值设定策略,实现自适应异常检测,降低了对完全依赖“分布内验证数据集”确定阈值的依赖。通过在多个数据集(包括合成与真实世界成像伪影、以及跨MRI序列的多种脑部病症)上进行的广泛评估,IterMask3D相较于现有方法,持续展现出更优或具有竞争力的性能。 广泛评估下的优异性能凸显了本方法的潜力,但仍存在若干挑战——其中许多是当前研究领域的共性问题,这些问题也为未来研究指明了有价值的方向。基于重建的异常检测方法普遍面临的一个挑战是:对于仅产生细微重建误差的异常,模型的检测灵敏度会降低。这类异常包括小型伪影或灰度强度接近均值的模式。加剧该问题的一个固有挑战是,训练与测试数据集之间可能存在潜在的领域差异(即成像特征的差异)。在实际应用中,很难使用与测试数据完全来自同一成像中心或同一台扫描仪的数据来训练模型。尽管我们提出的迭代式掩码优化方法,能在推理阶段通过向模型逐步暴露测试图像中更大的区域,来逐步缩小这种领域差异,但如果某些异常产生的重建误差与领域差异导致的重建误差相似,这些异常就可能被误判为正常区域。最终,这些异常在迭代过程中可能会被解除掩码,进而导致漏检。 另一个挑战在于,医学成像数据(尤其是MRI数据)的强度范围变异性与自然图像存在显著差异。自然图像通常具有固定的强度范围(如0-255),而MRI图像的强度范围在不同数据集、不同扫描仪和不同采集协议下差异极大——因为MRI强度单位尚未实现标准化。这种训练与测试分布间的显著强度偏移,会导致异常分割方法的性能不佳。与之相关的另一个挑战是医学图像强度归一化:该过程旨在为不同图像中的相同组织分配可比较的强度范围,以避免强度偏移对重建精度和异常检测产生负面影响。然而,MRI强度归一化仍是一个活跃的研究领域,现有归一化方法极易受到图像中异常区域的影响。因此,效果不佳的强度归一化会给异常检测或分割带来进一步的挑战。 尽管存在上述挑战,我们提出的IterMask3D方法仍为该领域做出了有意义的贡献:它关注灵敏度-精度权衡问题,并引入迭代式掩码缩小策略,在3D异常检测任务中有效缓解了这一矛盾。实验结果表明,该方法在成像伪影检测方面表现尤为突出,这对成像工作流中的质量保障具有实际应用价值。在无监督病症分割任务上,尽管该方法的性能已达到无监督分割领域的先进水平,但与有监督分割方法的性能仍有差距,存在进一步提升的空间。不过,本方法所展现出的改进潜力也表明,无监督异常分割领域仍存在大量未被挖掘的可能性。考虑到无监督方法在某些场景下(如各类脑部病灶分割或偶发异常发现)比有监督方法更具适用性——这类场景中,有监督方法因需要大量标注数据而难以应用——我们希望本研究能为该方向的未来研究提供启发与推动。
Results
结果
5.1. Synthetic artifact detection on 3D images
We first evaluate performance of our model for detecting artifactsthat may appear as unexpected anomalies during deployment. Methods for detecting unexpected artifacts during acquisition are usefulfor implementing quality-assurance frameworks. It is often difficult toannotate precise ground truth segmentation labels for artifacts, particularly when they manifest as global patterns across the entire image.Additionally, image-level detection is typically sufficient for identifyingsuch anomalies. Therefore we evaluate performance based on artifactdetection rather than segmentation.We begin by testing the model’s ability to detect synthetic artifacts.We train models on cognitively normal data from ADNI using the FLAIRsequence, which contain no pathology or artifacts. This defines the indistribution normal data. To evaluate anomaly detection, we first assessthe model’s ability to detect scans of a different sequence. Then, wesimulate various types of anomalies (anomalies closer to the trainingdistribution) in reference of Graham et al. (2023) and Ravi et al. (2024)by generating images with missing chunks in the middle or top regionsof the brain, adding Gaussian noise, adding spike noise, bias field,random ghosting, or zipper artifact as illustrated in Fig. 5 first row. Thesimulated anomalies are added to FLAIR from the ADNI validation set,while the original, unaltered images are used as in-distribution normalcontrol samples for performance evaluation. A detailed description ofeach artifact is provided below.
5.1 3D图像上的合成伪影检测 我们首先评估所提模型在检测部署过程中可能出现的意外伪影(表现为异常)方面的性能。在图像采集阶段检测意外伪影的方法,对构建质量保障框架具有重要意义。然而,为伪影标注精确的分割真值标签往往存在难度,尤其是当伪影在整幅图像中呈现全局分布模式时。此外,对于此类异常的识别,通常只需实现图像级别的检测即可。因此,我们基于伪影检测(而非分割)来评估模型性能。 我们首先测试模型检测合成伪影的能力。模型的训练数据来自ADNI数据集(FLAIR序列)中认知功能正常被试的图像,这些图像不含任何病症或伪影,构成了“分布内正常数据”。为评估异常检测效果,我们首先测试模型对不同序列扫描图像的检测能力。随后,参考Graham等人(2023)和Ravi等人(2024)的研究,我们模拟了多种类型的异常(与训练分布更接近的异常):生成脑部中间或顶部区域存在“缺失块”的图像、添加高斯噪声的图像、添加尖峰噪声的图像,以及存在偏置场、随机重影或拉链伪影的图像(如图5第一行所示)。这些模拟异常被添加到ADNI验证集的FLAIR序列图像中,而未经修改的原始图像则作为“分布内正常对照样本”,用于性能评估。各类伪影的详细说明如下。
Figure
图

Fig. 1. Sensitivity–Precision Trade-off in reconstruction-based methods:When corruption (e.g., noise or compression) is lower, the reconstructionerror over anomalous regions (the hyper-intense tumor) remains low, oftenresulting in missed detections. As corruption increases, errors in abnormalregions become more pronounced, improving sensitivity—but at the cost ofhigher errors in normal regions, which can increase false positives and reduceprecision
无监督异常检测与分割方法的优缺点有哪些? ## 图1 基于重建的方法中的“灵敏度-精度权衡” 当损坏程度(如噪声或压缩)较低时,异常区域(高信号肿瘤)的重建误差仍较低,往往会导致漏检。随着损坏程度的增加,异常区域的误差会更加显著,灵敏度随之提升——但代价是正常区域的误差也会增大,这可能导致假阳性增多并降低精度。 ## 图1 基于重建的方法中的“灵敏度-精度权衡” 当损坏程度(如噪声或压缩)较低时,异常区域(高信号肿瘤)的重建误差仍较低,往往会导致漏检。随着损坏程度的增加,异常区域的误差会更加显著,灵敏度随之提升——但代价是正常区域的误差也会增大,这可能导致假阳性增多并降低精度。
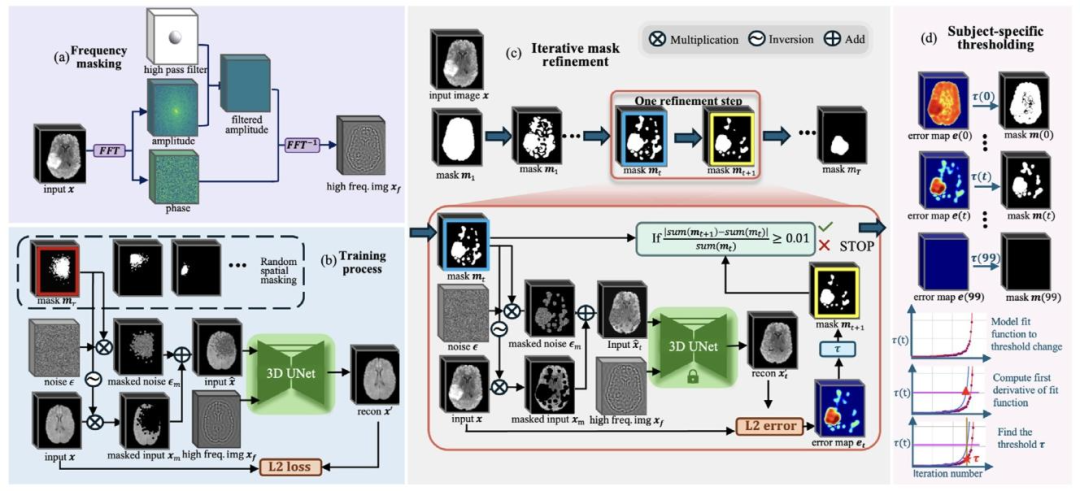
Fig. 2. Overview of the proposed approach. (a) Frequency-based masking: Extract structural information 𝑥𝑓 with a high-pass filter to isolate the high-frequencycomponents of the image. (b) Training process: Input image 𝑥 is masked using random spatial masking, and the model learns to reconstruct the masked area with𝑥𝑓* generated in (a) as an auxiliary input. © Iterative mask refinement: Iterative refinement of the spatial mask, where the mask 𝑚𝑡 at iteration 𝑡 gradually shrinkstowards the anomalous region. This refinement is guided by the spatially unmasked portions of the image 𝑥𝑚 and 𝑥𝑓 from (a). (d) Subject-Specific Thresholding:This component illustrates how the threshold 𝜏 is determined for iterative mask refinement on a per-sample basis. The mask is progressively shrunk by 1% ofthe brain volume at each time step 𝑡 until it fully disappears. We model 𝜏(𝑡) using a fitted function, and compute its derivative to identify the point where thethreshold begins to change abruptly. This point is selected as the sample-specific threshold 𝜏 for the current sample.
图2 所提方法概述 (a)基于频率的掩码:通过高通滤波器提取结构信息(x_f),以分离图像的高频成分。 (b)训练流程:采用随机空间掩码对输入图像(x)进行掩码处理,模型以(a)中生成的(x_f)作为辅助输入,学习对掩码区域进行重建。 (c)迭代式掩码优化:对空间掩码进行迭代优化,其中第(t)次迭代时的掩码(m_t)逐步向异常区域收敛。该优化过程由图像的未掩码部分(x_m)及(a)中生成的(x_f)共同引导。 (d)特定于被试的阈值设定:该模块展示了如何为每个样本的迭代式掩码优化确定阈值(\tau)。在每个时间步(t),掩码按脑体积的1%逐步缩小,直至完全消失。我们通过拟合函数对(\tau(t))建模,并计算其导数以确定阈值开始急剧变化的临界点,该临界点被选为当前样本的特定阈值(\tau)。

Fig. 3. Illustration of threshold dynamics with fixed shrinking speed of the mask. The figures shown are plotted from four randomly chosen samples, twofrom the BraTS FLAIR sequence (left) and two from the BraTS T2 sequence (right). For each sample, the mask is progressively shrunk by 1% of the brain volumeper iteration until it fully disappears. The purple points represent the threshold required to produce the current shrunken mask based on the reconstruction errormap, with the red curve showing the fitted function. Orange points indicate the corresponding Dice scores between the mask and the ground truth anomaly at eachiteration. As the mask approaches the anomaly, the Dice score increases and peaks when the anomaly is optimally exposed. Correspondingly, the correspondingthreshold rises gradually, then sharply increases near the anomaly boundary—indicating a distinct shift in reconstruction error and serving as a marker for findingthe optimal stopping threshold
图3 掩码固定缩小速度下的阈值变化动态示意图 所示图像源自4个随机选取的样本,其中2个来自BraTS FLAIR序列(左侧),2个来自BraTS T2序列(右侧)。对于每个样本,掩码在每次迭代中按脑体积的1%逐步缩小,直至完全消失。 紫色点代表根据重建误差图生成当前缩小后掩码所需的阈值,红色曲线为拟合函数;橙色点代表每次迭代中掩码与异常区域真值(ground truth)之间的对应Dice系数。随着掩码逐步向异常区域靠近,Dice系数不断升高,并在异常区域被最优暴露时达到峰值。与之对应,阈值先缓慢上升,在接近异常区域边界时急剧升高——这表明重建误差发生显著变化,同时也是确定最优停止阈值的标志。

Fig. 4. Illustration of the datasets used in the format: dataset name (sequence).First row, left to right, ADNI (FLAIR), OASIS (T2), Private Artifact dataset (T2);Second row, left to right, BraTS (FLAIR), BraTS (T2), BraTS (T1ce); Third row,left to right, BraTS (T1), ISLES (FLAIR) (with small lesion), ISLES (FLAIR) (withbig lesion); Fourth row, three samples from ADNI (FLAIR) with problematicskull-stripping
图4 所用数据集示意图(格式:数据集名称(序列)) 第一行从左至右:ADNI数据集(FLAIR序列)、OASIS数据集(T2序列)、私有伪影数据集(T2序列); 第二行从左至右:BraTS数据集(FLAIR序列)、BraTS数据集(T2序列)、BraTS数据集(T1ce序列); 第三行从左至右:BraTS数据集(T1序列)、ISLES数据集(FLAIR序列,含小病灶)、ISLES数据集(FLAIR序列,含大病灶); 第四行:ADNI数据集(FLAIR序列)中3个颅骨剥离存在问题的样本。
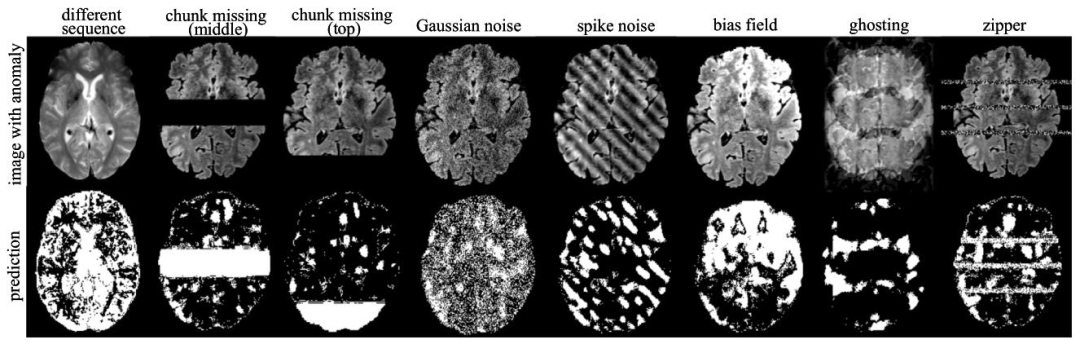
Fig. 5. Visualization of anomaly detection results on images with synthetic anomalies using our method 𝐼 𝑡𝑒𝑟𝑀𝑎𝑠𝑘3𝐷. The first row shows the input images withsimulated anomalies, while the second row displays the corresponding detected anomaly areas.
图5 采用所提方法IterMask3D对含合成异常图像的异常检测结果可视化 第一行展示含模拟异常的输入图像,第二行展示对应的检测到的异常区域。
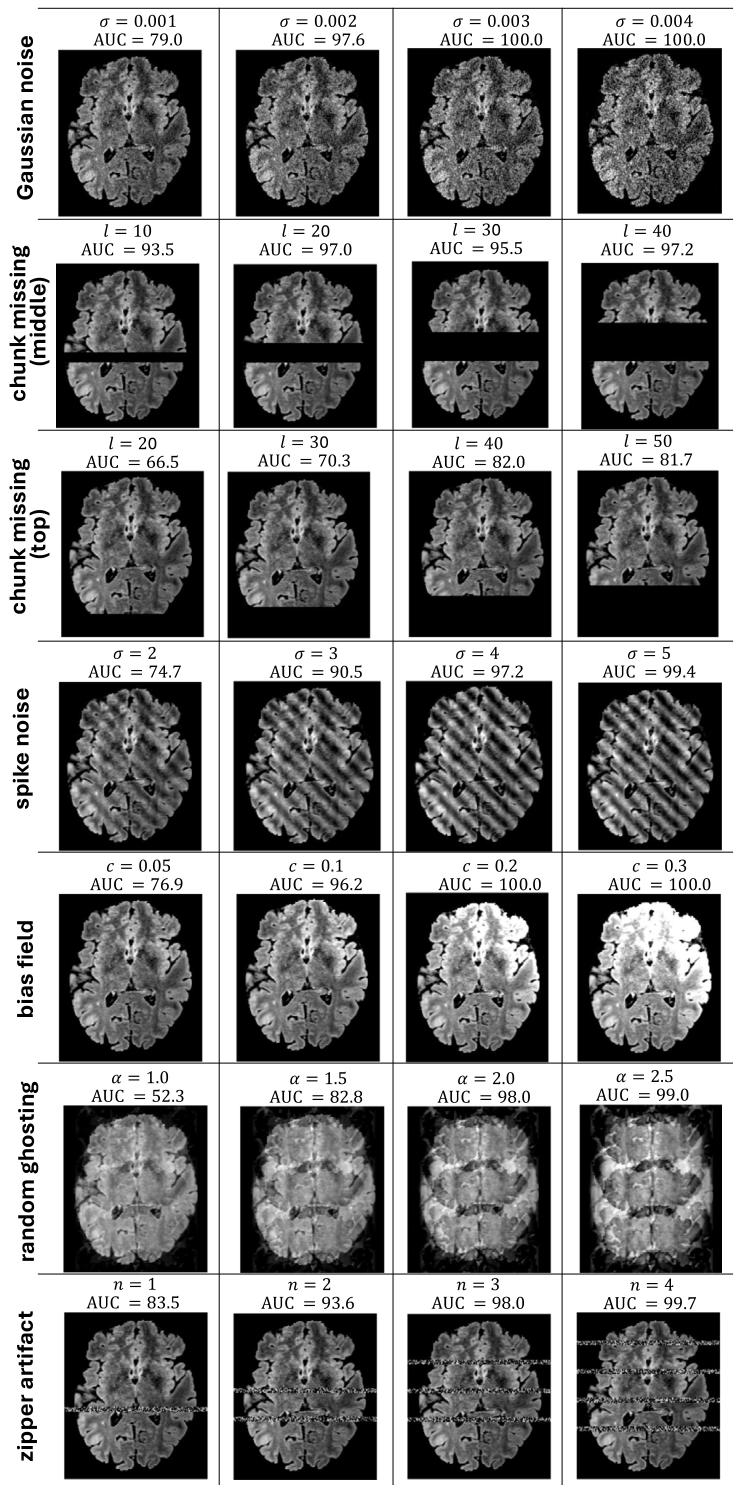
Fig. 6. Visualization of different extents of synthetic anomaly added to the image, along with 𝐴𝑈𝐶 score of the model’s anomaly detection performance.
图6 图像中添加不同程度合成异常的可视化结果,以及模型异常检测性能的AUC值
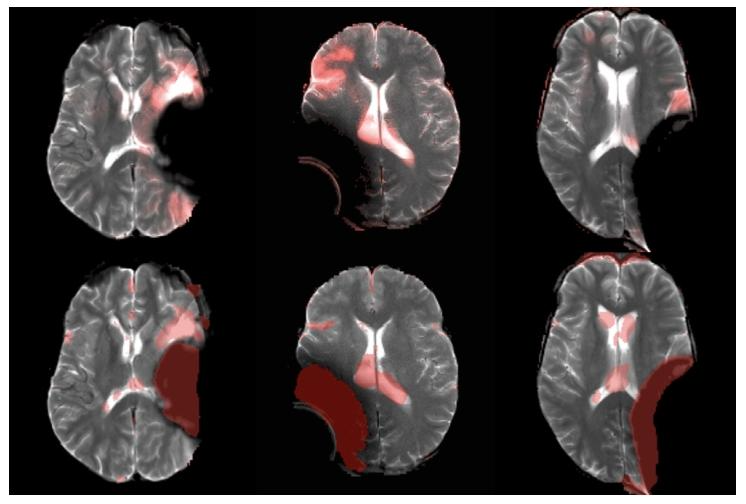
Fig. 7. Visualization of artifact detection results on our private dataset usingDAE (Kascenas et al., 2022) (top row) and our method 𝐼 𝑡𝑒𝑟𝑀𝑎𝑠𝑘3𝐷 (bottomrow). The final anomaly map is overlaid in red on the original image. DAEis shown to detect the hyper-intense area (mostly in normal brain area) asanomaly, while our method better detects the actual anomalous artifact.
图7 采用DAE方法(Kascenas等,2022)(上行)与所提方法IterMask3D(下行)在私有数据集上的伪影检测结果可视化 最终异常图以红色叠加显示在原始图像上。结果显示,DAE方法会将高信号区域(多为正常脑区)误检测为异常,而所提方法能更准确地检测出真实的异常伪影。

Fig. 8. Anomaly segmentation results on 3D FLAIR inputs from BraTS using 𝐼 𝑡𝑒𝑟𝑀𝑎𝑠𝑘3𝐷. Red contours indicate the boundaries of the predicted segmentationmasks overlaid on the original images.
图8 采用IterMask3D方法在BraTS数据集3D FLAIR序列输入图像上的异常分割结果 红色轮廓代表预测分割掩码的边界,该边界叠加显示在原始图像上。
Table
表

Table 1Performance for detecting anomalous synthetic imaging artifacts in 3D scans. Best in bold
表1 3D扫描中异常合成成像伪影的检测性能(最优结果以粗体标注)
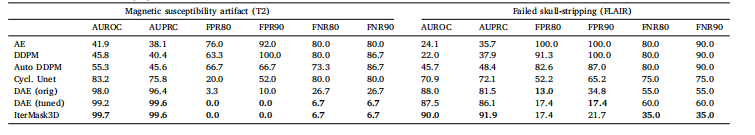
Table 2Detection of real-world imaging artifacts in 3D scans. Best in bold
表2 3D扫描中真实世界成像伪影的检测性能(最优结果以粗体标注)

Table 3Performance of methods for pathology segmentation on 2D slices across all MRI sequences in BraTS. Best in bold.
表3 各方法在BraTS数据集所有磁共振成像(MRI)序列的2D切片上的病症分割性能(最优结果以粗体标注)

Table 4Performance for 3D pathology segmentation in BraTS and ISLES datasets. Bestin bold.
表 4 BraTS 与 ISLES 数据集上 3D 病症分割性能(最优结果以粗体标注)
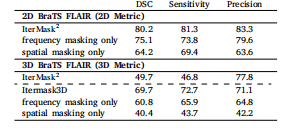
Table 5Ablation study for observing the effect of spatial and frequency masking in the 2D (top) and 3D (bottom) versionsof our model. We also show performance of our 2D model(IterMask2 ) in 3D images from BRATS (bottom) for straightforward comparison with IterMask3D, showing the gainsfrom 3D modeling.
表5 空间掩码与频率掩码作用的消融实验结果 (上表为模型2D版本的实验数据,下表为模型3D版本的实验数据) 此外,为与IterMask3D进行直观对比(以体现3D建模的优势),表中还展示了2D模型(IterMask2D)在BraTS数据集3D图像上的性能表现。
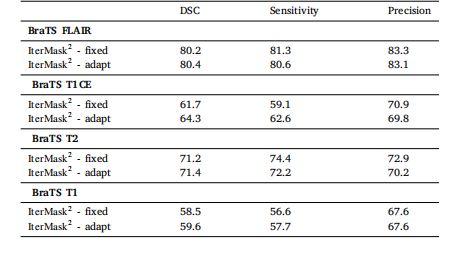
Table 6Ablation study of thresholding strategy with 𝐼 𝑡𝑒𝑟𝑀𝑎𝑠𝑘2 on 2D anomalysegmentation
表6 基于IterMask2D在2D异常分割任务中对阈值设定策略的消融实验

Table 7Ablation study of thresholding strategy with 𝐼 𝑡𝑒𝑟𝑀𝑎𝑠𝑘3𝐷 on 3Dreal-world anomaly detection.
表7 基于IterMask3D在3D真实世界异常检测任务中对阈值设定策略的消融实验